COPAL® G+C
Effectiveness in revision surgery.
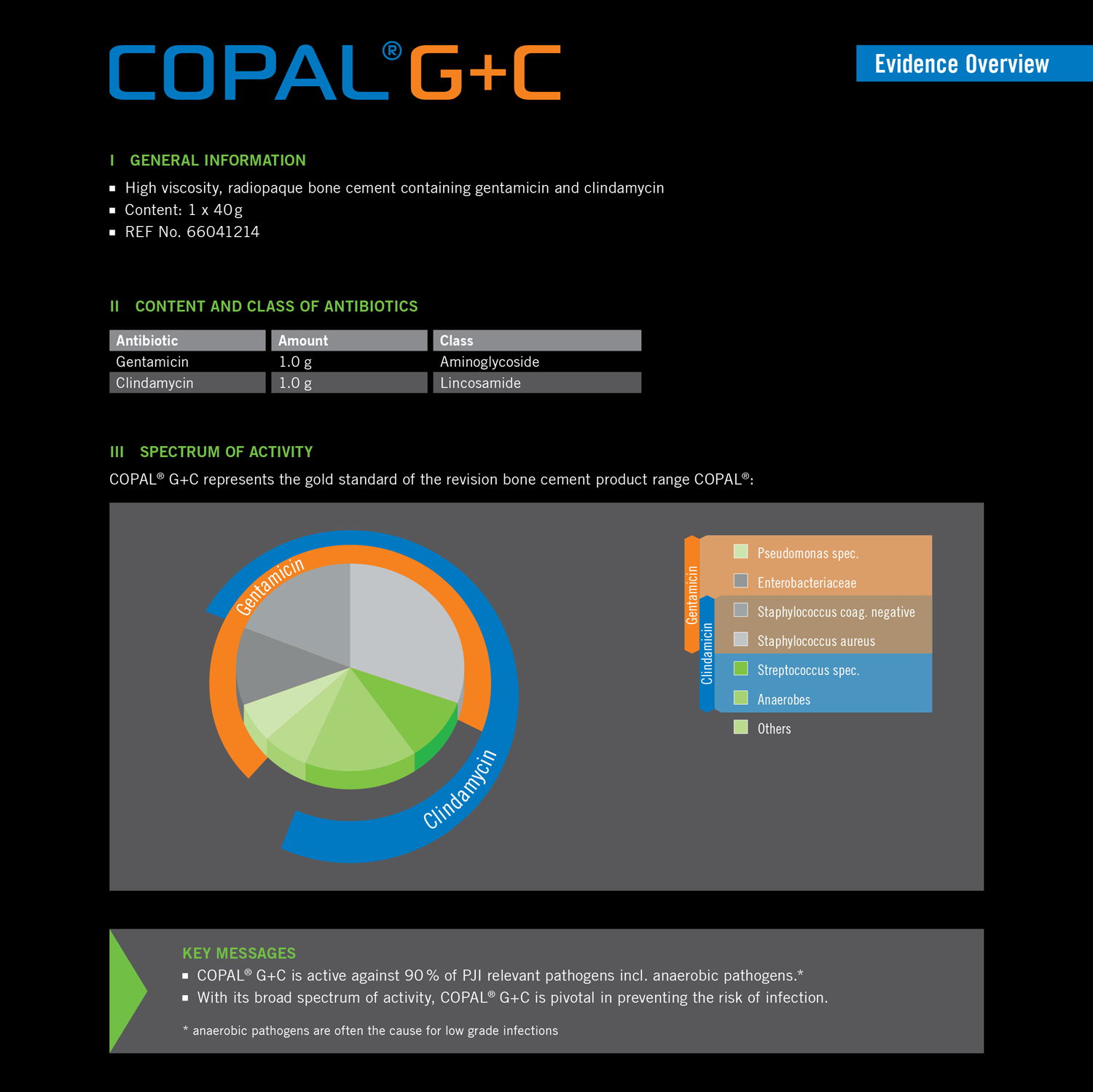
COPAL® G+C is a high-viscosity bone cement for anchoring prosthetic implants in revision surgery. It contains the antibiotics gentamicin and clindamycin. This combination offers a broad spectrum of activity against around 90% of clinically-relevant pathogens. Clindamycin specifically targets anaerobic pathogens and offers a high degree of bone penetration. COPAL® G+C supports a very high initial local concentration of antibiotics. This means that a build-up of resistance can be minimised. The result is effective protection of the prosthetic implant against bacterial colonisation and the formation of biofi lm, with minimal systemic exposure for patients (2).
RELIABLE ACROSS THE BOARD
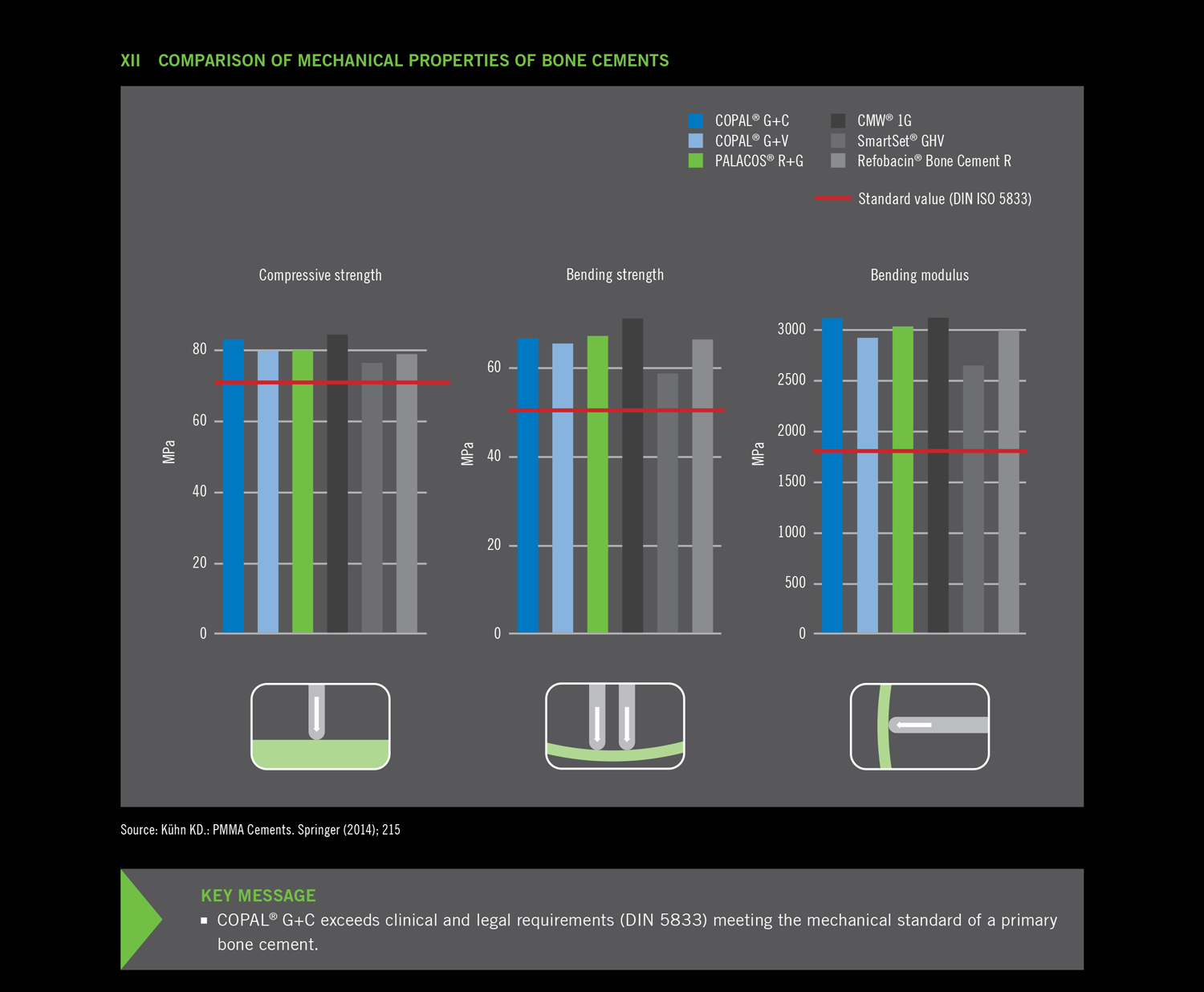
Due to the combination of gentamicin and clindamycin, COPAL® G+C meets the requirements for an effective revision surgery concept. The use of COPAL® G+C for prevention of infection is also recommended in the cases of revision surgery procedures that are considered aseptic in which the risk of late infection as a result of slow-growing pathogens (known as low-grade infection) cannot be completely excluded (3).
SIMPLE TO USE
COPAL® G+C offers consistent quality due to industrial production. COPAL® G+C supports an effi cient workflow as well as the minimization of the potential for errors.

KEY MESSAGES
COPAL® G+C is active against 90 % of PJI relevant pathogens incl. anaerobic pathogens.*
With its broad spectrum of activity, COPAL® G+C is pivotal in preventing the risk of infection.
- anaerobic pathogens are often the cause for low grade infections
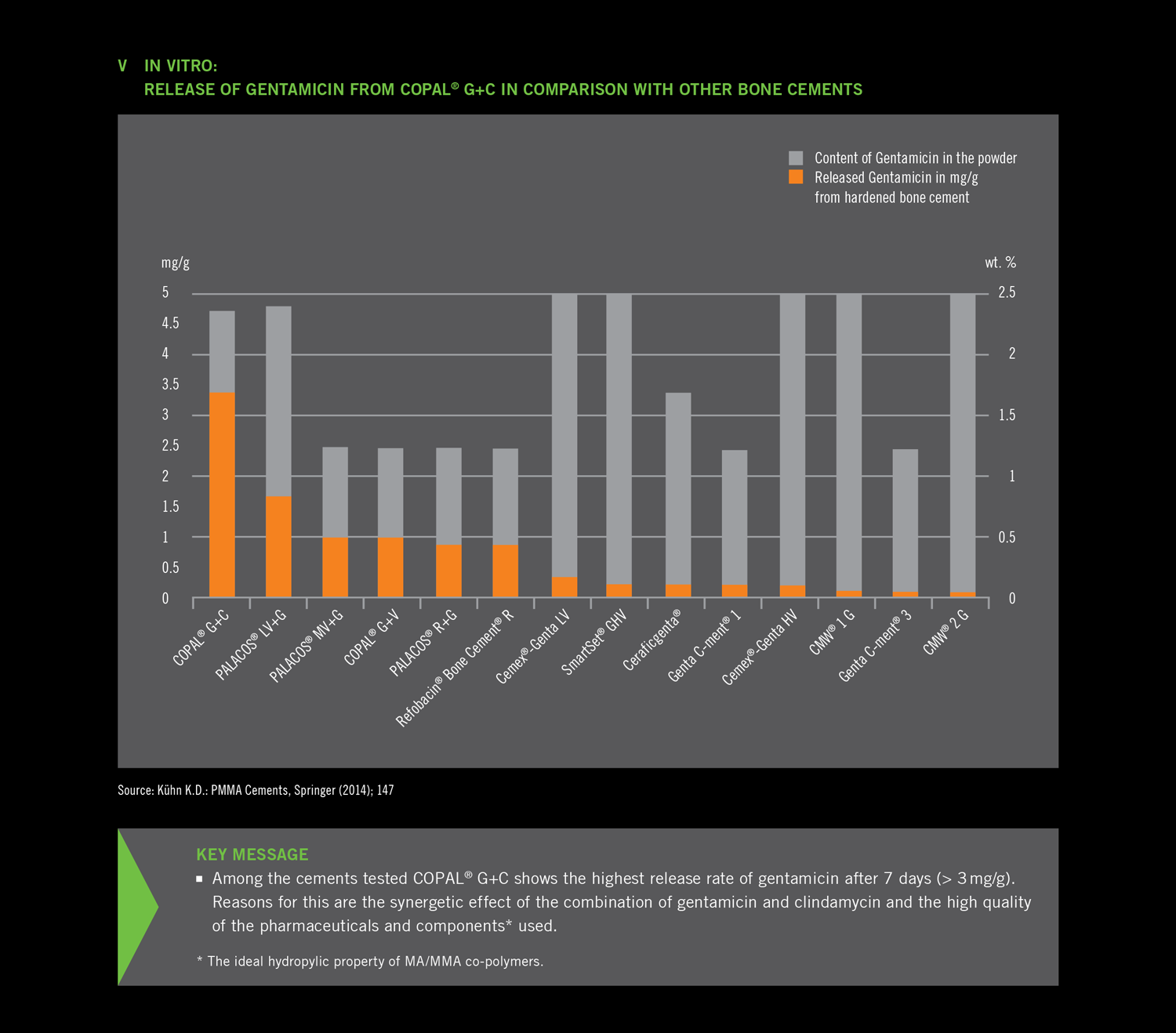
- The combination of gentamicin and clindamycin shows a synergetic effect resulting in an increased release of
both antibiotics. - Among the cements tested COPAL® G+C shows the highest release rate of gentamicin
after 7 days (> 3 mg/g). Reasons for this are the synergetic effect of the combination of gentamicin and clindamycin and the high quality
of the pharmaceuticals and components* used. - The ideal hydropylic property of MA/MMA co-polymers.
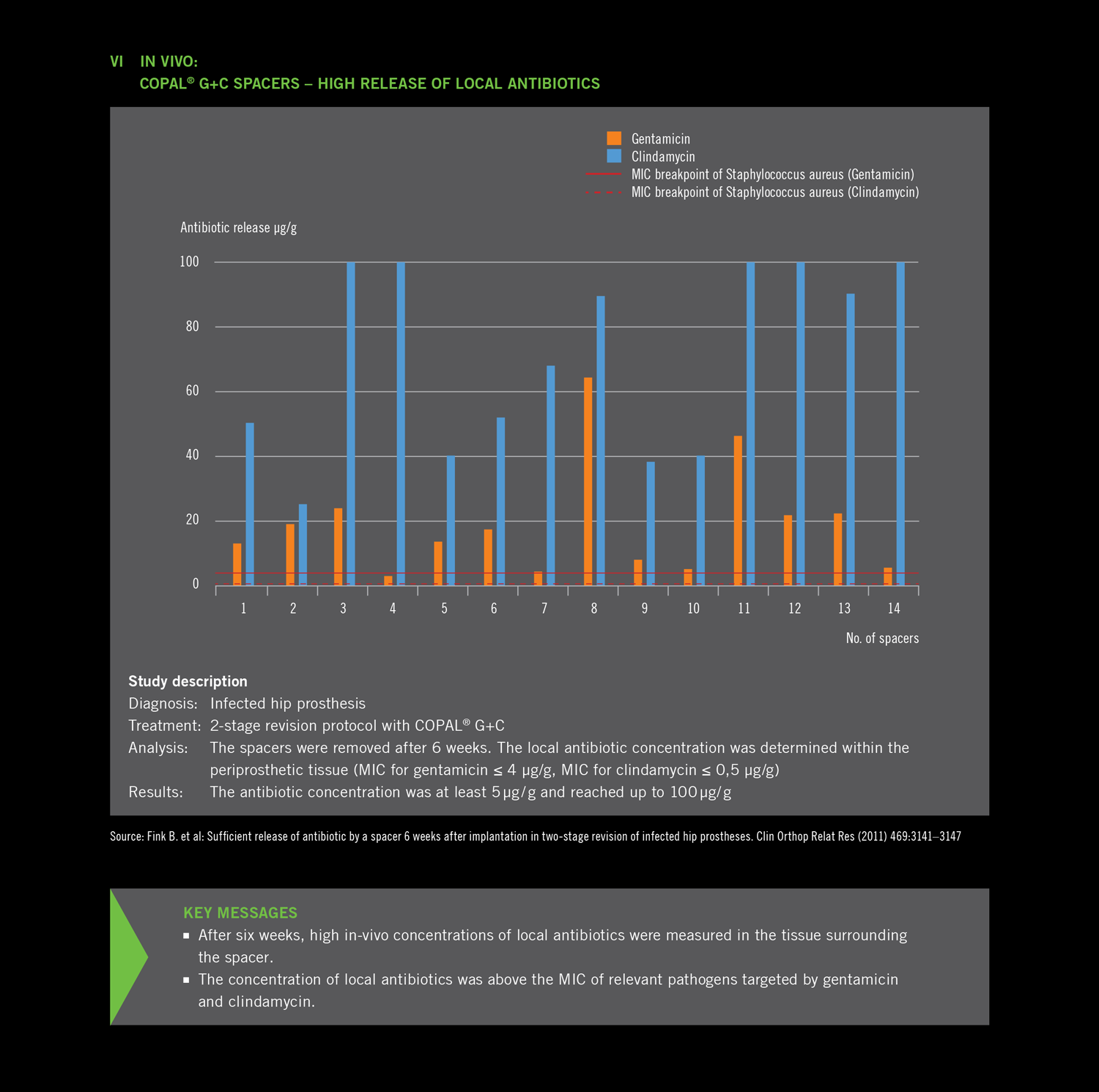
- After six weeks, high in-vivo concentrations of local antibiotics were measured in the tissue surrounding
the spacer.