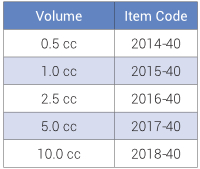
Allograft
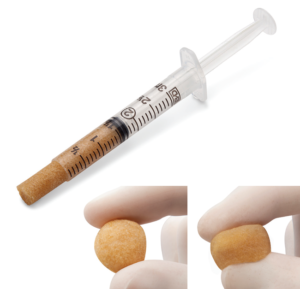
DBM Putty
- 100% Allograft
- Moldable, resistant to irrigation that provides the maximum amount of DBM
- All lots tested for BMP and osteoinductivity
- Demineralized process validated for viral inactivation
Safety Assurance
Maxxeus DBM Putty is packaged in a double, sterile pouch in a plunger. Each graft has a three year expiration date and stored at ambient temperature. Grafts are prepared using a patented bioburden reduction process, which when combined with terminal irradiation provides a Sterility Assurance Level of 10-6. All donors are recovered and processed in the United States of America.
Osteoinductive
Demineralized Bone Matrix (DBM) contains a variety of biologically active bone morphogenic proteins (BMPs), which are among the osteoinductive components of bone. More than 20 BMPs have been identified in human allograft bone tissue. A validated in vitro osteoinductive assay using ELISA BMP 2 concentration measurement method is used to verify that BMP is present in our final DBM product.

